Chemistry is key to keeping your pool clean, as well as making it a safe, pleasant environment for swimming.
Chemicals like chlorine are used to keep algae and bacteria under control, while chemicals like muriatic acid keep the pH levels of your pool’s water balanced. You’ll need test strips and a drop kit to check and maintain chlorine and pH levels. It also is important to keep a consistent volume of water in your pool; a lower-than-normal level can make your chemicals too intense and a higher-than-normal level can dilute them and make them ineffective. Improper water levels will also have detrimental effect on filtration and circulation.
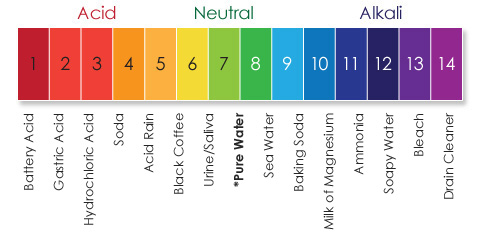
The ideal range for pH in swimming pool water is 7.0 – 7.6. The pH of our eyes is typically 7.2 – 7.4. In our experience, if the pH is kept at the same level as that in our eyes, the side-effects of burning red eyes is kept to a minimum. The ability of chlorine to disinfect at this level is also optimal.
What is pH?

High pH in swimming pool water may cause the following problems:
- calcium buildup on pool surfaces, waterline and accessories
- dull or cloudy pool water
- clogging of filter medium or elements
- drop in disinfection potential of chlorine resulting in algae growth
- burning eyes and nose
- dry, itchy skin and scalp
Lowering High pH in Pool Water
Adding an acid to the pool water reduces the pH. The most common chemicals used to reduce high pool water pH are:
- muriatic acid — typically 30% – 35% liquid hydrochloric acid
- sodium bisulfate — granule or powder pH reducer, dry acid
Other acids that have been used in pool water are:
- sulfuric acid — raises TDS levels and adds sulfates to the pool water
- nitric acid — highly corrosive but is known to work well
Alkalinity

If the Total alkalinity of the pool water is within the recommended parameters of 80 – 120ppm, pH reducer should be added according to the instructions on the container. The acid should usually be added to water and mixed before dosing the pool. The pump should be running when the acid is slowly distributed around the pool. Low pH in swimming pool water may cause one or more of these problems:
- eroding of the pool plaster or grouting
- corroding of the metal pool accessories (steps, heater, etc.)
- staining resulting from metal corrosion
- rapid dissipation of chlorine requiring increased dosage
- burning eyes and nose
- dry, itchy skin and scalp
- perishing of swimwear, pool toys and accessories
Raising Low pH in Pool Water
Adding a base or alkalinity raises the pH of the pool water. If the total alkalinity is normal, pH increaser should be added according to the instructions on the container. The active ingredient is usually sodium carbonate. Often low pH is a result of acid rain and occurs after periods of heavy precipitation. The normal tendency of pool water pH is to rise through exposure to wind, sunshine and bathers. The most common cause of consistently low pH is low total alkalinity, which should always be adjusted (with sodium bicarbonate) before trying to increase the pH.